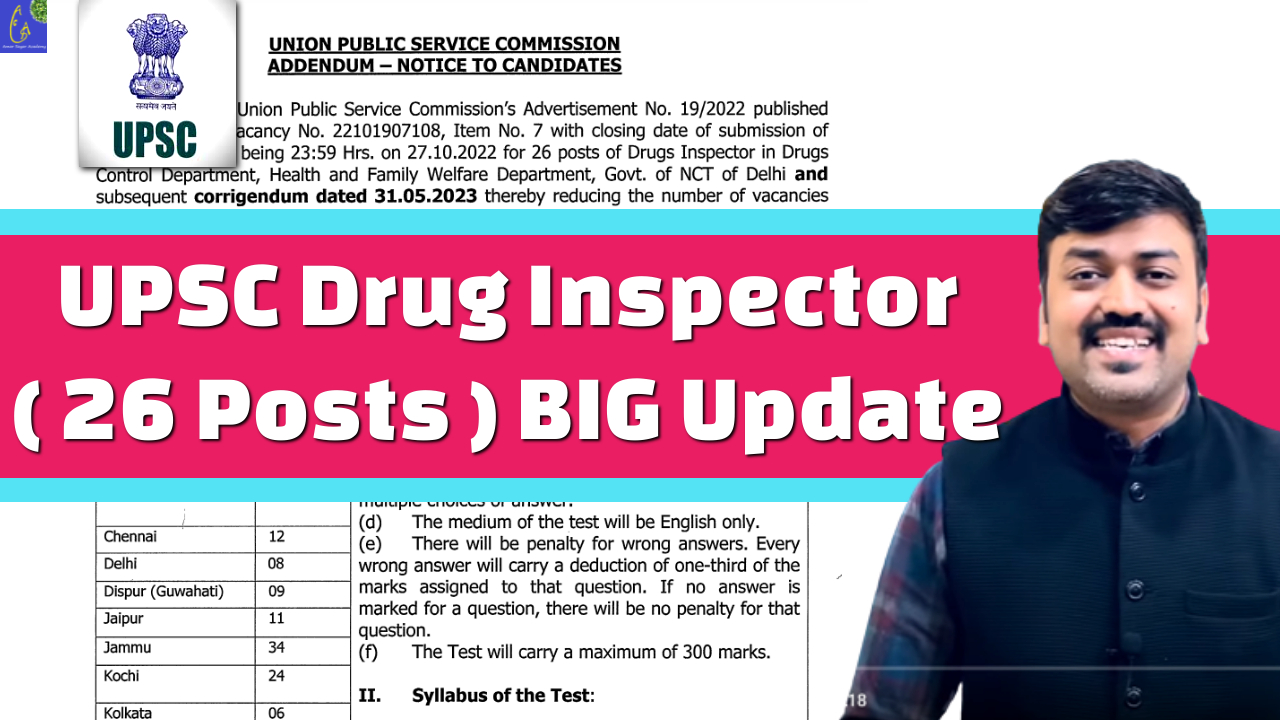
Big Update Admit Card Released on 01/08/2023
Introduction: The Union Public Service Commission (UPSC) has recently announced the examination date for the Drugs Inspector recruitment for the year 2023. Aspiring candidates who wish to pursue a career in the pharmaceutical industry and contribute to ensuring drug safety can now mark their calendars for the 19th of August, 2023. Additionally, the UPSC has released the detailed syllabus for the examination, providing candidates with valuable information to prepare effectively.
Exam Date: 19th August 2023 The UPSC has scheduled the Drugs Inspector examination to be held on the 19th of August, 2023. This highly anticipated exam will be an excellent opportunity for candidates looking to secure a prestigious position as a Drugs Inspector, responsible for maintaining the quality and safety of drugs in the country. Aspiring candidates now have a clear target date to focus their preparations and ensure they are well-prepared for the challenges ahead.
Syllabus Overview: To assist candidates in their exam preparations, the UPSC has released the detailed syllabus for the Drugs Inspector examination. The syllabus covers various aspects related to pharmaceuticals and drug control, ensuring a comprehensive assessment of the candidates’ knowledge and skills. Here is a brief overview of the key subjects included in the syllabus:
- Pharmaceutical Chemistry: This section focuses on topics such as drug synthesis, drug analysis, drug stability, pharmaceutical impurities, and pharmaceutical additives.
- Pharmacology: Candidates will be tested on their understanding of drug actions, drug interactions, adverse drug reactions, pharmacokinetics, and pharmacodynamics.
- Pharmaceutical Analysis: This section deals with analytical techniques used in drug testing, including instrumental methods like chromatography, spectroscopy, and validation of analytical procedures.
- Pharmaceutical Jurisprudence: Candidates will be evaluated on their knowledge of drug laws, drug regulations, and various acts and rules related to the pharmaceutical industry in India.
- Microbiology: This section covers various aspects of microbiology relevant to pharmaceuticals, such as microbial contamination, sterilization, disinfection, and microbial quality control.
- Quality Assurance: Candidates will be assessed on their understanding of quality control, quality management systems, Good Manufacturing Practices (GMP), and Quality Assurance (QA) in the pharmaceutical industry.
- Pharmaceutical Technology: This section focuses on topics related to dosage forms, pharmaceutical product development, stability testing, and pharmaceutical packaging.
Conclusion: The announcement of the UPSC Drugs Inspector examination date on the 19th of August 2023 has set the stage for aspiring candidates to gear up their preparations. With the release of the detailed syllabus, candidates can now plan their studies and concentrate on the key subjects outlined by the UPSC. The Drugs Inspector position holds significant responsibility in ensuring the safety and quality of drugs in the country, making this examination a crucial step towards a rewarding career in the pharmaceutical industry. Candidates are encouraged to utilize the available time wisely and prepare thoroughly to increase their chances of success in the examination.